One Study, Multiple Answers
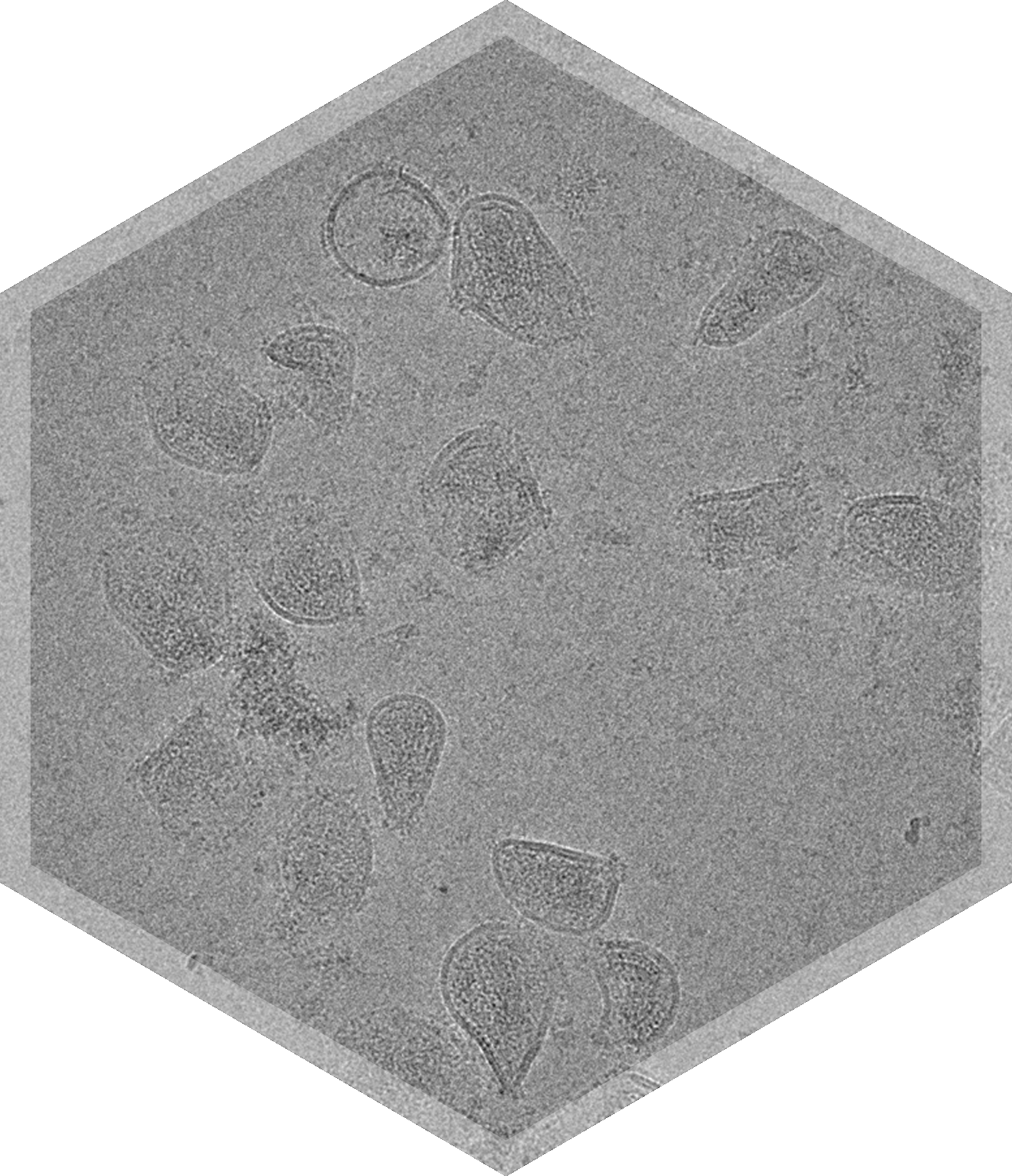
Cryo-TEM imaging is a vital technique for nanoparticle characterization, providing detailed quantitative and qualitative analysis of your formulation with just one study.
Cryo-TEM & TEM imaging reveal Critical Quality Attributes (CQAs) and structural details that other methods miss, using only a small amount of sample.
From R&D through GMP, we provide

Accelerate Drug Discovery with Cryo-TEM
Cryo-TEM can unveil many aspects of a sample in just one study, including particle size distribution, drug encapsulation, shape, morphology, impurities, and integrity, all with only ~50μl of sample.
Cryo-TEM is a powerful orthogonal technique to complement your nanoparticle characterization toolkit.

Cryo-TEM & TEM for Nanoparticle Characterization
Crucial Insights into Your Formulation
Characterize Your Nanoparticles
Therapeutic nanoparticles are complex, and can vary in composition, shape, and size.
Nanoparticle size, shape, lamellarity and morphology affect drug incorporation, stability, and release, which in turn affect cell toxicity, targeting, and therapeutic efficacy. In addition, the study of drug cargo encapsulation when looking at gene or drug delivery systems is crucial, as the presence of many empty particles or impurities affects immunogenic response.
Cryo-TEM & negative stain TEM can evaluate multiple CQA's with one study.
Our Nanoparticle Experience
AAV samples imaged; 3D structure of AAV solved to 1.8 Å
Liposomal samples imaged, including LNP, EV, and OMV projects
Virus and VLP samples imaged

Cryo-TEM in Action

Frequently Asked Questions
Is cryo-TEM imaging with NIS GMP compliant?
Can I see an example report of nanoparticle characterization and analysis?
What can cryo-TEM tell me about my nanoparticles or nanomedicines?
Why should I use cryo-TEM instead of Dynamic Light Scattering (DLS) to measure particle size?
Why should I use cryo-TEM instead of analytical ultracentrifugation sedimentation velocity (AUC-SV) to measure full/empty capsids for samples like Adeno-associated viruses (AAVs)?
Why should I use cryo-TEM in addition to other RNA encapsulation methods?
How is cryogenic transmission electron microscopy (cryo-TEM) different than transmission electron microscopy (TEM)?
What kind of samples do you have experience with?
What is the size range of nanoparticles you can image with cryo-TEM?
What is the likelihood for success in characterizing my nanoparticle sample?
What quantity of my sample is required?
Are all reagents and buffer conditions amenable to cryo-EM?
What are the turnaround times to receive images and a report?
Does NIS handle documentation or paperwork?