Per-Particle Characterization of Vesicular Stomatitis Virus
Vesicular Stomatitis Virus (VSV) is gaining ground as a versatile platform in oncolytic therapy and vaccine development due to advantages such as the lack of pre-existing immunity in humans, its high potency, and ease of genetic engineering.
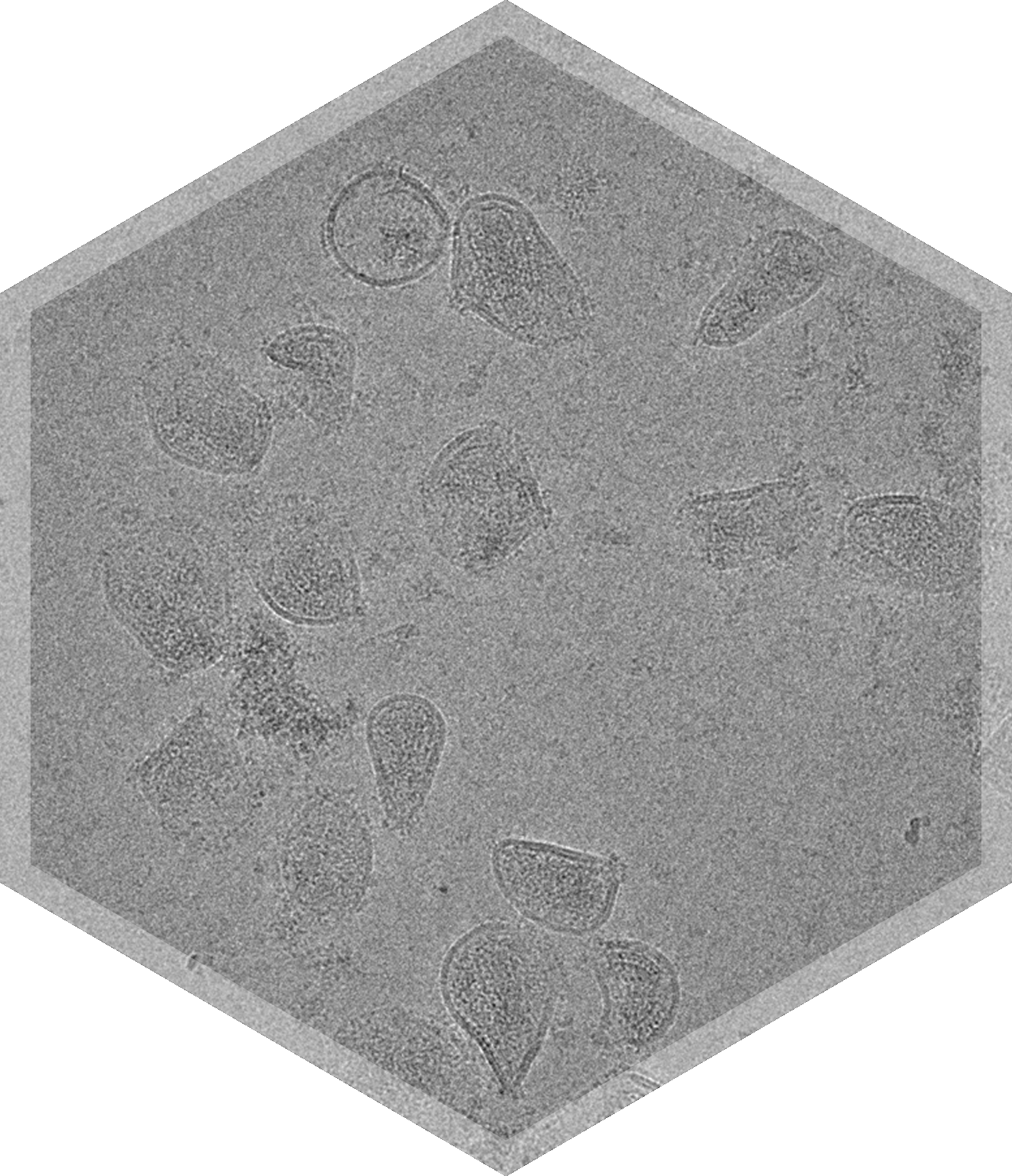
VSV’s characteristic bullet shape and its surface glycoprotein arrangement affect its interaction with host cells and viral fusion efficiency and therefore have a direct impact on infectivity and efficacy. VSV’s size and aggregation state are important factors in cellular uptake and immunogenicity.
Characterization of the morphology, size, aggregation status, purity, surface glycoprotein integrity, and overall structural integrity of VSV particles is crucial to ensure consistency in safety and efficacy of VSV formulations used in oncology, vaccines, and gene therapies. Cryo-TEM imaging provides direct, high-resolution visualization of VSV particles in their near-native fully hydrated state, allowing researchers to assess multiple quality attributes simultaneously and make more informed decisions.
Direct Visualization of VSV
Cryo-TEM imaging & image analysis can reveal unique insights about VSV-based therapeutics such as:
- Characteristic bullet-shaped morphology of VSV particles
- Distribution of glycoprotein spikes on the VSV surface
- Structural defects, such as broken capsids or malformed virions
- Aggregation status
- Individual particle dimensions and size distribution
- Overall homogeneity and purity

Viral & VLP Formulations Related to VSV

Characterization of VSV with Cryo-TEM
Vesicular stomatitis virus (VSV), an enveloped negative-stranded RNA virus, has emerged as a powerful platform across vaccines, oncology, and gene therapy. VSV’s RNA genome encodes five structural proteins: nucleoprotein, phosphoprotein, matrix protein, glycoprotein, and viral polymerase. Viral entry and exit, as well as cellular recognition and fusion, are facilitated by the glycoprotein, whereas the matrix protein is essential for formation of the viral core and anchoring of the glycoprotein to the viral membrane. Cell transcription is performed by RNA-dependent RNA polymerase, which is driven by a nucleoprotein, viral polymerase, and phosphoprotein complex. The virus’s relatively simple molecular structure and rapid replication cycle alongside the lack of pre-existing immunity in humans and ease of genetic engineering have made VSV a powerful tool in precision medicine.
As a vaccine vector, VSV can be genetically engineered to express antigens from other viruses (e.g., Ebola, SARS-CoV-2) on its surface, stimulating strong humoral and cellular immune responses. As an oncolytic virus, VSV benefits from a rapid replication cycle that enables efficient tumor cell lysis before host immune responses can mount significant antiviral activity, and it harbors natural selectivity for cancer cells, especially those with defective interferon signaling. For gene therapy, VSV's broad tropism and cytoplasmic replication allow for transient expression of therapeutic genes with minimal risk of genome integration. To ensure safety, efficacy, and consistency in these applications, critical quality attributes (CQAs) of VSV must be carefully characterized.
Cryo-TEM is a powerful analytical tool that can be used to assess VSV at high resolution in its near-native state. This technique provides direct visualization of viral particles embedded in vitreous ice, without staining or fixation, preserving their morphology and structure with nanometer-scale detail. Cryo-TEM imaging is especially valuable in providing critical insights into the following VSV CQAs:
- Size and morphology influence biodistribution, cell entry, and immune recognition - Cryo-TEM allows for precise measurement of VSV’s characteristic bullet-shaped geometry, including length, width, and curvature. This is critical to confirm identity and monitor consistency across batches.
- Purity and aggregation can compromise safety and efficacy – Cryo-TEM can discern debris and proteins, and can detect and quantify particle clumping or fusion events, which other techniques like DLS might miss or underestimate.
- Surface glycoprotein distribution is essential for infectivity, immunogenicity, and targeting - High-resolution imaging can show the density and arrangement of surface glycoproteins (e.g., VSV-G).
- Integrity of capsid and genomic core structure ensures that the virus remains functional – Cryo-TEM reveals structural damage or incomplete particles, such as broken envelopes or core degradation, which are key indicators of product stability and quality.
Cryo-TEM is uniquely suited to assess the structural CQAs of VSV that directly impact its performance and safety. By enabling direct, visual verification of viral morphology and composition, cryo-TEM supports quality control, process optimization, and regulatory compliance throughout VSV therapeutic development.
Frequently Asked Questions
How many particles do you count, or images do you analyze per sample or grid?