NIS collaborated with scientists from Frontier Medicines to conduct structural validation of DCAF2.
We are proud of our contributions to the characterization of DCAF2 as a promising E3 adaptor for PROTAC strategies.
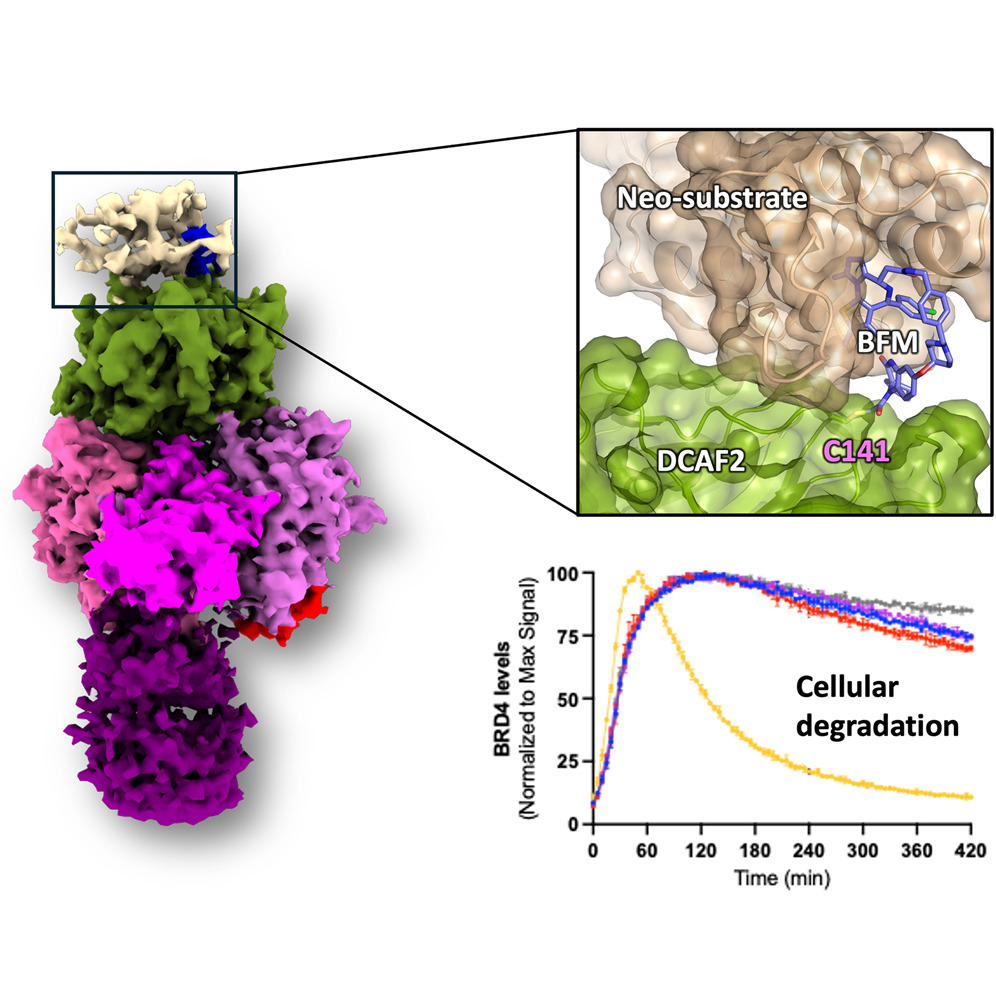
In “Structural basis for DCAF2 as a novel E3 ligase for PROTAC-mediated targeted protein degradation,” NanoImaging Services collaborated with scientists from Frontier Medicines to conduct structural validation of DCAF2, a Cullin4-RING ligase substrate adaptor implicated in DNA damage response and cancer, as a novel E3 for targeted protein degradation.
Using cryo-EM, structures of the DCAF2:DDB1:DDA1 complex (3.3 Å), a ligand-bound complex (3.1 Å), and a ternary complex with a covalent PROTAC and BRD4 (3.4 Å) were solved, revealing PROTAC-mediated substrate recruitment.
These findings expand the repertoire of E3 ligases suitable for TPD, broadening therapeutic applications and opening avenues for the development of tumor-selective cancer therapies.
We are proud of our contributions to the characterization of DCAF2 as a promising E3 adaptor for PROTAC strategies.
In “Structural basis for DCAF2 as a novel E3 ligase for PROTAC-mediated targeted protein degradation,” NanoImaging Services collaborated with scientists from Frontier Medicines to conduct structural validation of DCAF2, a Cullin4-RING ligase substrate adaptor implicated in DNA damage response and cancer, as a novel E3 for targeted protein degradation.
Using cryo-EM, structures of the DCAF2:DDB1:DDA1 complex (3.3 Å), a ligand-bound complex (3.1 Å), and a ternary complex with a covalent PROTAC and BRD4 (3.4 Å) were solved, revealing PROTAC-mediated substrate recruitment.
These findings expand the repertoire of E3 ligases suitable for TPD, broadening therapeutic applications and opening avenues for the development of tumor-selective cancer therapies.
Structural basis for DCAF2 as a novel E3 ligase for PROTAC-mediated targeted protein degradation, McMahon, Evan J. et al., Structure, Volume 0, Issue 0